Fluorescense and Phosphorescense

What is fluorescence
When we look this up at Wikipedia (https://en.wikipedia.org/wiki/Fluorescence), we get the following explanation:
“Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. The most striking example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the spectrum, and thus invisible to the human eye, while the emitted light is in the visible region, which gives the fluorescent substance a distinct color that can only be seen when exposed to UV light.”
Well, that all sounds a bit complicated. I’ll try to explain a bit:
All matter around us is build up by atoms. Well knows atoms are:
- Oxygen ( O )
- Hydrogen ( H )
If atoms get combined, molecules are created, for example:
- Oxygen ( O2 ) . It is striking that the name for the molecule Oxygen is the same as for the atom Oxygen. Actually the Oxygen (O2) we breath is di-oxygen, also called molecular Oxygen. With O2 there are 2 “O” atoms combined together.
- Water ( H2O ). In this case 2 Hydrogen (H) atoms are bound to 1 Oxygen (O) atom
Atoms themselves exist of a nucleus (which exists of protons and neutrons) and around this nucleus circle electrons. And its these electrons that it’s all about with fluorescence.
Below a picture of an Oxygen atom:
In the middle of the atom is the atomic nucleus, around this nucleus circle the electrons.
These electrons turn round in a sort of balance around the nucleus. This balance can in some cases be disturbed by UV-light.
When the energy of UV-light (light exists of fotons, the shorter the wavelength, the more energy it contains) hits the electrons, some electrons get elevated to a higher orbit where there are also other electrons.
This condition cannot be held and the electron will return to its original position. During this return energy is released in a small amount of heat and in the form of visible light
Below a picture of this process:

Lightspectrum
The lightcolor our eye can see is from deep red to deep purple. Below are two diagrams that show this:

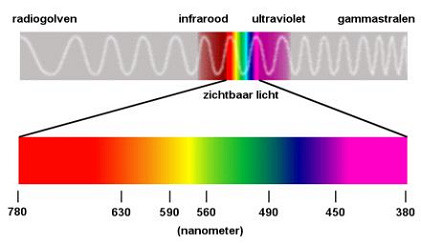
As one can see there is infrared before red and ultra violet after violet. The shorter the wavelength, the more energy it contains.
Ultraviolet light
At wavelengths shorter than 400 nanometers , we speak of ultraviolet light. This range can be devided in UV-A, UV-B and UV-C radiation.

With fluorescent minerals we generally make a distinction between UV-A, UV-B and UV-C active minerals. There is a large number of minerals which fluoresce under the influence of UV-A light. Wellknown examples are Ruby and Fluorite .
Beside these there are a lot of minerals that fluoresce under UV-C light. This is less know/used because of the special lamps with special filters that are needed to see this phenomenon .
Many of these minerals also fluoresce under UV-B light, sometime the fluorescence is in another color
This is what makes fluorescent minerals so attractive. The diversions in fluorescence are very wide and fluorescent minerals occur in all the colors of the rainbow.
